An analysis conducted on the results of a recently published review by CoEHAR experts has, for the first time, assessed the quality of reporting on oral adverse events related to the use of smoke-free products. The findings highlight that many trials present fragmented and incomplete information, undermining the ability to reliably evaluate the tolerability of these products.
n international research group, coordinated by CoEHAR in Catania, examined data from 36 randomized clinical trials included in a review recently published by the same team. The aim was to comprehensively assess, for the first time, the quality of the information concerning oral adverse events linked to the use of modified-risk products.
The analysis revealed the absence of specific classification systems for oral events, making accurate evaluation difficult.
“The first point of contact between smoke-free devices and our body is the oral cavity,” explains Giusy La Rosa, author of the study and CoEHAR researcher. “There are cases where these products can cause adverse events, often mild or transitory in nature. To properly assess tolerability and the associated risk profile, it is essential that oral adverse events are reported within studies precisely and according to updated standards. For example, the appearance of ulcers in the oral cavity could result not only from product use but also from smoking cessation and associated physiological changes. The lack of precise temporal data prevents distinguishing between product effects and withdrawal-related phenomena. And the same goes for other adverse effects.”
One of the main problems identified is the lack of ad hoc classification systems for oral adverse events: the current scales may lead to inconsistent or underestimated reporting.
This study represents a milestone in assessing the quality of oral adverse event reporting in trials on smoke-free products for smoking cessation, and it underlines the need to involve oral health professionals in trial design to accurately identify and classify such outcomes.
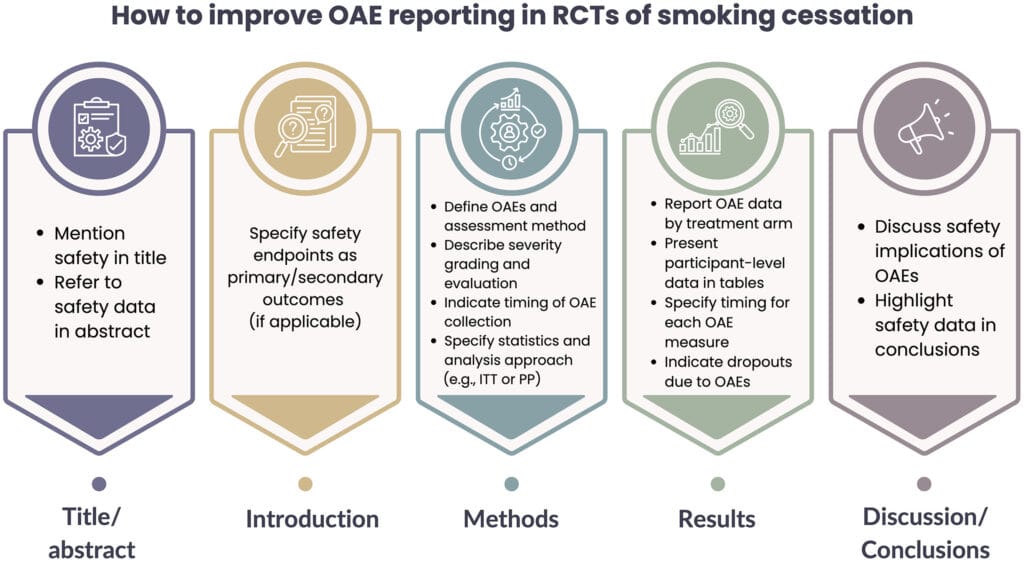
The analysis also includes a series of practical recommendations to improve reporting:
- clearly mention safety in the title and abstract;
- provide precise definitions of adverse events and severity criteria;
- report data collection methods, timing of assessments, and statistical approaches;
- present disaggregated data for each trial arm and discuss clinical implications in a balanced way.
As noted earlier, this research analyzes the data of a large-scale systematic review aimed at evaluating the health effects of various modified-risk, smoke-free products. The review compared results against placebo, traditional smoking, and the potential impact on health treatments undergone by study participants.
The findings show that most smoke-free products are generally well tolerated and do not increase the risk of oral health adverse effects such as irritation, ulcers, or dryness. Reported adverse events are often mild to moderate and transitory, and very rarely lead users to discontinue oral health treatments.
“The aim was to assess the real effects of modified-risk products on oral health, relying on the broadest body of evidence available to us,” explains La Rosa. “Although the data confirm that these tools can be a valid aid in mitigating the negative effects of smoking on the mouth, it emerged that the oral adverse events considered in studies on alternative products (nicotine replacement therapy [NRT], electronic cigarettes, …) for smoking cessation are reported inconsistently and often with insufficient detail. Therefore, a meaningful comparison remains difficult, greatly limiting the possibility of introducing these products into clinical practice or including them in the broader debate on public health policies.”
In summary, smoke-free products appear to be generally well tolerated, though not entirely free of adverse effects in specific cases.
The adoption of the proposed recommendations is crucial to improving transparency, guiding cessation research, and strengthening the evidence base for harm reduction policies, while also enhancing the role of oral health professionals in supporting patients throughout their cessation journey.